Abstract
Introduction: Acute lymphoblastic leukemia (ALL) represents approx. 5% of all newly diagnosed leukemias in patients between 55 and 70 years of age. Despite recent advances especially in younger patients, the prognosis of elderly patients with ALL remains dismal, even after moderately intensive chemotherapy. Incorporation of antibody-based therapies in the first line induction therapy bears the potential of a significant increased response rate and survival with the reduction of treatment toxicity.
Methods: This open label phase II study of the German Multicenter Study Group on Adult Acute Lymphoblastic Leukemia was conducted at 13 centers. Patients aged >55 years with newly diagnosed Ph/BCR-ABL negative acute B-precursor ALL were eligible. Leukemic blasts had CD22 surface expression of at least 20%. The 1st induction cycle consisted of InO 0.8mg/m2 on day1 and 0.5mg/m2 on d8 and d15 together with dexamethasone 10 mg/m2 (day7-8, day14-17) and one intrathecal injection (i.th.) of methotrexate (MTX), cytarabine (AraC) and dexamethasone (Dex). The 2nd and 3rd induction cycle consisted of InO 0.5mg/m2 on day 1, 8 and 15 plus i.th. MTX/AraC/Dex. Patients achieving a complete remission (CR) were offered to receive 5 conventional consolidation therapies (3 x ID-MTX/asparaginase; 2 x ID-AraC) and one reinduction therapy (idarubicine/ AraC/ cyclophosphamide / Dex.) in combination with rituximab (for CD20+ ALL), followed by a maintenance therapy with 6-MP/MTX. The primary endpoint was event free survival (EFS) at 12-months follow-up. An event was defined as any of the following: persisting bone marrow blasts after 2 cycles of InO, relapse or death. An event rate of ≤40% at 12 months follow-up was considered to qualify the experimental treatment as very promising for additional testing. Under the assumption of one-sided type I error of 5% and 80% power, 42 evaluable patients were needed for primary endpoint analysis (registered with ClinicalTrials.gov, identifier: NCT03460522).
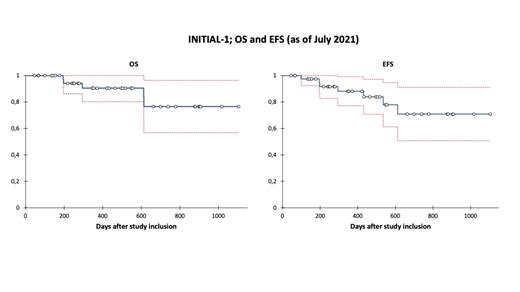
Results: As of July 2021, 45 patients were included, with induction results available for 43 patients. Two patients were excluded per protocol from further analysis, as they received only one induction: 1 prolonged toxicity (CR after induction), 1 withdrawn consent. Median age at initial diagnosis was 64 years (range 56-80 years). 38 patients were diagnosed with a common- and 5 with a pro-B ALL. Median CD22 expression on leukemic blasts was 69% (range 21-99%). Due to suspected therapy related liver toxicities, a single patient received only 2 induction cycles (complete remission after 1st induction). All other patients completed 3 cycles of induction therapy and achieved complete remission (CR/CRi), mainly after the 1st induction. Results of minimal residual disease (MRD), measured by real-time quantitative PCR, are available for all 43 patients, with 23/43 (53%) and 31/42 (74%) patients being MRD negative after 2nd and 3rd induction therapy, respectively. Four patients with MRD failure (≥10 -4) after the 3rd induction therapy are still in CR (2 were treated with conventional chemotherapy per this protocol, one proceeded to allogeneic stem cell transplantation (aSCT), one received blinatumomab). MRD below the threshold of 10 -4 was detected in 15 patients after the 2nd induction (no relapse occurred so far). 8 of these 15 patients were MRD negative after the 3rd induction. So far, 7 events have been reported (during year 1: 3 deaths in CR and 1 relapsed ALL; during year 2: 1 death in CR and 2 patients with relapse). With a median follow-up of 422 days, the probability of OS at 1 and 2 years is 91% (95% C.I. 80-100%) and 77% (95% C.I. 57-96%), respectively. 4 patients received an aSCT in 1st CR, 1 patient in 2nd CR. Regarding adverse events (AEs) during induction cycles 1, 2 and 3, most common AEs ≥CTC grade 3 reported were leukocytopenia (in 60%, 12% and 3% of all cases, respectively), anemia (28%, 2%, 0%), thrombocytopenia (35%, 7%, 3%) and elevation of liver enzymes (14%, 5%, 0%). So far, one patient has been reported with suspected veno occlusive disease (after 2nd induction therapy).
Conclusion: Three cycles of InO as induction therapy for the treatment of elderly patients with acute B-cell ALL results in very high remission rates with MRD-response in up to 90% (MRD negativity or MRD-levels <10 -4). The promising EFS and survival rates of these patients warrant further investigation of this novel induction therapy in a prospective randomized trial.
Stelljes: Pfizer: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Medac: Speakers Bureau; MSD: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Kite/Gilead: Consultancy, Speakers Bureau. Wäsch: Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Consultancy; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Consultancy; BMS/Celgene: Consultancy; Gilead: Consultancy. Nachtkamp: Jazz: Honoraria; Bsh medical: Honoraria; Celgene: Other: Travel Support. Haenel: Jazz: Consultancy, Honoraria; GSK: Consultancy; Bayer Vital: Honoraria; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Amgen: Consultancy; Celgene: Consultancy, Honoraria. Berdel: Philogen S.p.A.: Consultancy, Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees. Goekbuget: Amgen: Consultancy, Other: Invited talks for company sponsored symposia (with honoraria); Research funding for institution; Astra Zeneca: Other: Invited talk for company sponsored symposia (with honor); Erytech: Consultancy; Cellestia: Consultancy; Incyte: Other: Research funding for Institution; Jazz Pharmaceuticals: Other: Research funding for institution; Novartis: Consultancy, Other: Research funding for Institution; Gilead/Kite: Consultancy; Morphosys: Consultancy; Pfizer: Consultancy, Other: Research funding for institution; Servier: Consultancy, Other; Abbvie: Other.
Inotuzumab Ozogamicin

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal